Researchers of CAMP-Nano uncover the mystery of oxygen embrittlement in Body-Centred-Cubic Niobium
Refractory body-centred-cubic (BCC) metals are widely used in nuclear energy, heating elements and rockets due to their high melting temperature, excellent strength and creep resistance and good compatibility with liquid metals. However, their mechanical properties rapidly degrade in the presence of dilute impurities such as O, C and N. Niobium (Nb), for instance, has appreciable oxygen solubility, and hardens and embrittles at low oxygen levels, decreasing its malleability and fracture toughness and limiting its applications. While the underlying mechanism is still a puzzle in this field, researchers from CAMP-Nano has uncovered the hardening and damage initiation mechanism underlying the embrittlement phenomenon, which provides clear defect evolution process from the atomic scale to macroscopic scale.
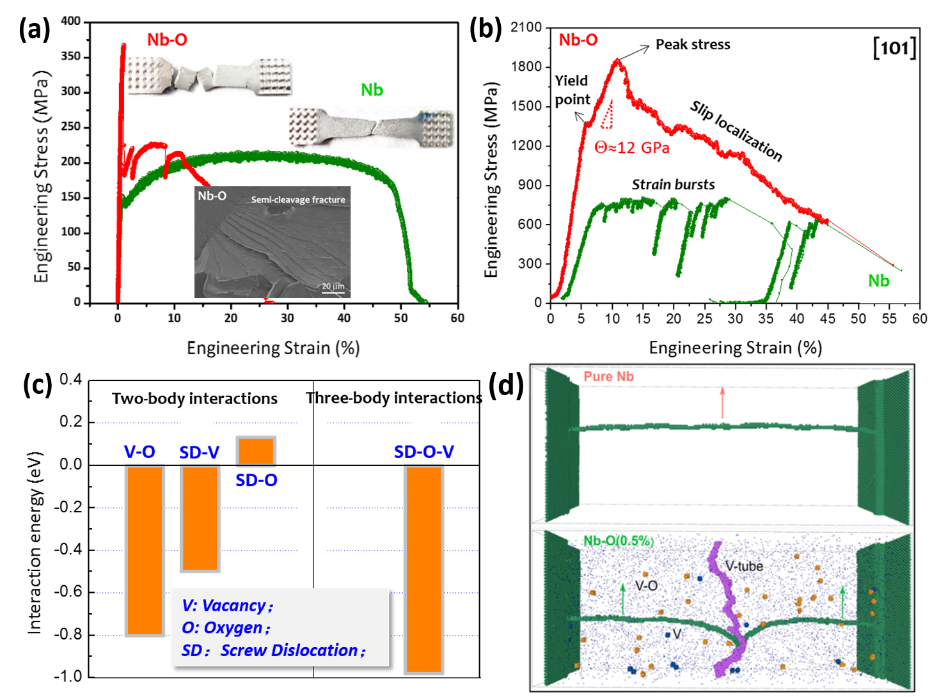
By macro-scale tensile test, researchers find that only 1 at.% solute oxygen can greatly embrittle niobium(Nb) while dramatically increasing its yield strength (figure(a)). Further fracture surface analysis reveals that the embrittlement behavior of niobium with 1 at.% solute oxygen (Nb-O) was caused by localized slip induced quasi-cleavage fracture. For a comprehensive view of fracture process, micro-tensile test was employed. As shown in figure (b), high yielding point and distinct strain hardening stage with ultrahigh strain hardening rate (Θ>10 GPa) can be observed in Nb-O. In this stage, high density of dislocations are stored in Nb-O, which is different from the “mechanical annealing” effect seen in Nb. Afterwards, slip localization sets in and nano-cavities/cracks take the shape, which causes the final fracture. However, the role of solute oxygen and its interaction with screw dislocation that cause the hardening and nano-cavity formation is still a mystery.
For this circumstance, DFT simulations have been utilized to analyze the atomic scale interaction between oxygen solute and dislocation. It is found that the interaction energy between oxygen solute and screw dislocation is repulsive, thus screw dislocation can not been directly pinned by oxygen interstitials. However, as shown in figure (c), oxygen solute has a high affinity to vacancy to form vacancy-oxygen complex (V-O). While for the three-body interaction among vacancy (V), oxygen interstitial (O) and screw dislocation (SD), the attraction energy is even higher. This means it is the V-O complexes that lock down the SDs and contribute to the dramatic hardening.
While there is no excess vacancies in Nb-O, how can this pining effect work? By this, MD simulation was conducted with results shown in figure (d). The initial repulsive random force filed (RFF) imposed by O interstitials on SDs facilitates the formation of cross-kinks to generate excess defects such as vacancies, V-O complexes and self-interstitials (SIs) at a low stress. Upon the passing of ensuing SDs, SIs can be easily annealed out while most of the V-O complexes remain stable. These dynamically formed V-O complexes are obstacles for SDs and their average spacing is very small (<2 nm), and are much more difficult to be “picked up” by the dislocations compared to the SI clusters. Thus, dramatic hardening caused by the pinning effect of V-O complexes occurs. In terms of initiating damage, the biased vacancy accumulation at V-O provides the seed to form nano-cavities that can coalesce to form micro-cracks and precipitously decrease the apparent stiffness of the region due to geometrical softening, which caused slip localization and fracture.
While our model case is O in Nb, the defect mechanisms uncovered are likely to be relevant not only to O-embrittlement but also to generic damage problems including irradiation and H-embrittlement, when SDs are important for plasticity such as in BCC materials.
This work has been published on Acta Materialia, https://www.sciencedirect.com/science/article/pii/S1359645419301168?dgcid=author. This work was supported by National Key Research and Development Program of China, the National Natural Science Foundation of China, the 111 Project of China, and etc.

